In a series of announcements made in April and May, the Food and Drug Administration (FDA) has granted approval to several animal drugs targeting conditions like congestive heart failure in dogs and respiratory diseases in cattle and swine. Among these approvals is the first generic torsemide oral solution designed for managing pulmonary edema in dogs.
Torsemide for Dogs
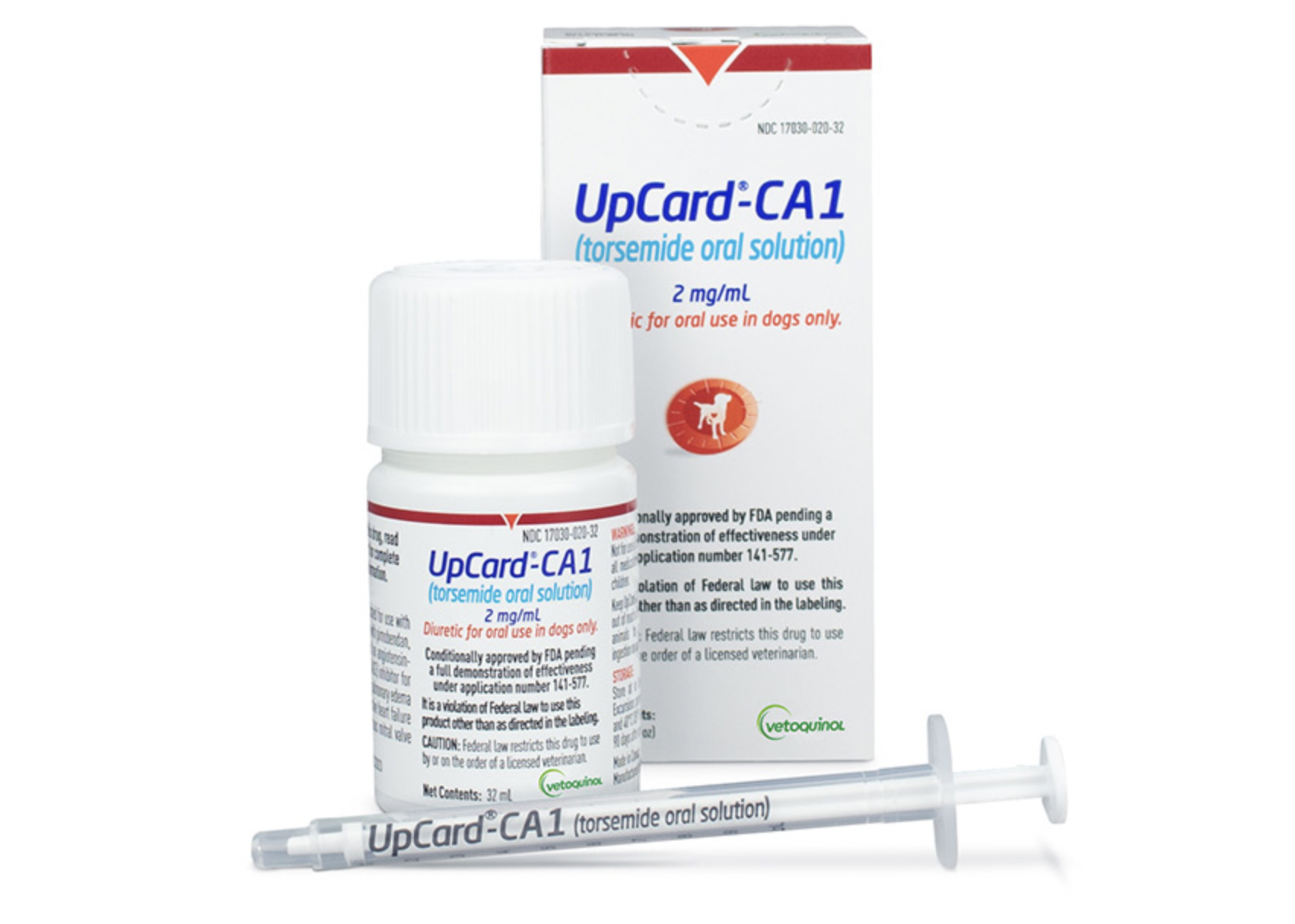
The FDA has conditionally approved UpCard-CA1, the first torsemide oral solution specifically for dogs suffering from pulmonary edema due to congestive heart failure caused by myxomatous mitral valve disease (MMVD). UpCard-CA1 is to be administered alongside pimobendan, spironolactone, and an angiotensin-converting enzyme inhibitor for optimal results.
MMVD is a serious and potentially life-threatening condition in dogs if left untreated. Given the severity, the FDA has deemed UpCard-CA1 eligible for expanded conditional approval. This initial approval is valid for one year and may be renewed annually up to four times, provided that the drug sponsor actively works toward collecting additional effectiveness data required for full approval.
Dosage and Availability:
- UpCard-CA1 is available in 32- or 96-mL vials.
- Administered orally once daily at a dose of 0.11 to 0.44 mg/kg (0.05 to 0.2 mg/lb) of body weight.
- Sponsored by Vetoquinol USA Inc., based in Fort Worth, Texas.
Pimomedin for Dogs
The FDA has also approved Pimomedin, a generic pimobendan chewable tablet for managing symptoms of congestive heart failure in dogs due to clinical MMVD or dilated cardiomyopathy (DCM). Pimomedin is designed for use in conjunction with other heart failure therapies such as furosemide.
Dosage and Availability:
- Available in 1.25, 2.5, 5, and 10 mg oblong half-scored chewable tablets, with 50 tablets per bottle.
- Administered orally at a total daily dose of 0.5 mg/kg (0.23 mg/lb) of body weight.
- Sponsored by Cronus Pharma Specialities India Private Ltd., based in India.
Pradalex for Cattle and Swine
The FDA has approved Pradalex, a pradofloxacin injection solution for treating certain respiratory diseases in cattle and swine. Pradalex is a third-generation fluoroquinolone that is critically important and can only be prescribed by a licensed veterinarian.
Cattle and Swine Respiratory Diseases:
- Approved for treating bovine respiratory disease (BRD) in specific ages and classes of cattle associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis.
- Also approved for treating swine respiratory disease (SRD) associated with Bordetella bronchiseptica, Glaesserella (Haemophilus) parasuis, Pasteurella multocida, Streptococcus suis, and Mycoplasma hyopneumoniae.
Usage Restrictions:
- For cattle: Approved for slaughter and breeding cattle less than 1 year of age.
- For swine: Approved for weaned swine intended for slaughter, not for breeding or nursing piglets.
Dosage and Availability:
- Supplied in 100- and 250-mL bottles, with each mL containing 200 mg of pradofloxacin.
- Administered as a single subcutaneous injection in cattle at a dosage of 10 mg/kg (4.54 mg/lb) of body weight and by intramuscular injection in swine at 7.5 mg/kg (3.4 mg/lb) of body weight.
- Sponsored by Elanco, based in Greenfield, Indiana.
These new drug approvals signify important advancements in veterinary medicine, offering new treatment options for managing serious health conditions in animals.